Introduction
Ketogenic diets have been shown to confer benefits in several pathological conditions including systemic (Westman et al., 2007; Pérez-Guisado, 2007) as well as neurologic (Kossoff, 2004) disorders. In theoretical papers, carbohydrate-restricted diets have repeatedly been suggested as a promising therapeutic approach in tumors (Ely, 1996; Klement and Kammerer, 2011; Seyfried, 2011, 2012). However, until now no more than a few studies reported on the use of the ketogenic diet in cancer patients.
Case studies with a positive effect include a landmark paper from 1995 (Nebeling et al., 1995) reporting of long-term survival of two children diagnosed with malignant brain cancer. Another case report indicated halted progression of glioblastoma multiforme while on the ketogenic diet (Zuccoli et al., 2010). Two group studies (Schmidt et al., 2011; Fine et al., 2012) reported stable disease in those cancer patients with sustained ketosis, however, both studies were limited in duration with three and one month's follow-up, respectively. In a recent study with patients with recurrent glioblastoma no significant clinical effect was seen (Rieger et al., 2014).
The benefits of the ketogenic diet are often explained evolutionarily (Fine et al., 2008). A few studies also report clinical advantages of the human evolutionary diet itself also known as the stone age diet (Voegtlin, 1975) or paleolithic diet (Cordain, 2002). Recently we have published two cases: one with childhood absence epilepsy (Clemens et al., 2013) and another with type 1 diabetes mellitus (Tóth and Clemens, 2014). Both patients were successfully treated with the paleolithic ketogenic diet and are still disease-free. Herein we present a case of bronchial tumor successfully treated with the paleolithic ketogenic diet.
Case report
The 58-year-old patient presented with shortness of breath, coughing, chest pain, and fatigue. Her previous medical history included right upper lobectomy of the lung due to bronchioalveolar carcinoma in 2003. The disease was staged as T2,N0,M0. In 2005 she was diagnosed with myoma and a hysterectomy was performed. In 2006 cholecystectomy was performed due to gall stones. In 2013 she was diagnosed with nodular goiter. In recent years the patient had recurrent upper and lower respiratory tract infections. The patient also had asthma bronchiale and high blood pressure. She reported no current and past smoking and alcohol abstinence. She was working as a singing teacher but her respiratory symptoms prevented her from working.
A cervical CT on 13 December 2013 revealed a pleiomorphic mass measuring 5 cm in diameter involving the lower segment of the trachea, bifurcation and the right bronchus. The mass narrowed the right bronchus to one-third. A CT examination of the chest on 23 December 2013 revealed no other abnormalities (Fig.1.). Only native CT scans were made because the patient’s thyroid disease prevented the use of a contrast medium.
On 19 December 2013 bronchoscopy confirmed imaging data and showed the stenosis of the right main bronchus. The tumor was biopsied and histopathological examination showed non-small cell lung cancer (NSCLC) adenocarcinoma. The patient was staged as T1,N0,M0. The patient was considered as being not eligible for surgery.
At this point, the patient consulted with the authors of the present paper who advised the paleolithic ketogenic diet. The patient initiated the diet on 20 January 2014. Her diet consisted of meat, offal, fat and eggs with a fat:protein ratio of at least 2:1. She did not consume vegetables, fruits, vegetable oils, dairy, and foods with additives. Coffee consumption was limited to a single coffe a day. No artificial sweeteners were allowed but she was allowed to use small amounts of honey for sweetening coffee. She was also taking 10,000 IU of vitamin D3 daily. No other supplements were allowed. She regularly checked ketosis using a urinary ketone test which indicated stable ketosis. The patient gave frequent feedbacks on weight, food records, and symptoms. Compliance was also checked by frequent laboratory work-ups.
When we first met the patient she was taking seven medications including valsartan, hydrochlorothiazide, allopurinol, nebivolol, aceclofenac, furosemide, and potassium chloride. She was also taking a combination of budesonide and formoterol as an inhalation aerosol. Upon diet onset, all medications were discontinued promptly except for nebivolol which was discontinued gradually within two weeks.
Along with the diet the patient received four cycles of chemotherapy (bevacizumab, paclitaxel, and carboplatin) between 16 January and 30 April 2014. Due to side effects, the patient asked for a waiver of further chemotherapy cycles following the fourth cycle.
A control CT on 21 March 2014 showed minor regression in the size of the tumor and increased diameter of the lumen of the right main bronchus (Fig.1.). To control for possible metastases MRI of the skull and whole-body bone scintigraphy were performed on 15 May 2014 and on 22 May 2014, respectively. These examinations showed no abnormalities.
A CT examination performed on 20 May 2014 showed further minor regression (Fig.1.). A PET-CT on 04 June 2014 showed pathologic FDG accumulation at the site of the tumor. Outside the tumor, no pathological FDG activity nor enlarged lymph nodes were noted.
At the time of diet onset, the patient’s weight was 78 kg which gradually decreased to 58 kg by October 2014. Thus her BMI decreased from 30.5 to 22.6 within 10 months. Laboratory workup was performed 13 times while on the diet. This showed that glucose levels were between 3.9 and 5.6 mmol/l (mean±SD=4.6±0.5 mmol/l). Urinary ketone was checked seven times by laboratory test and was positive on five occasions. Laboratory assessment showed leukocytopenia and anemia while on chemotherapy. Following the cessation of chemotherapy, cell counts normalized and the elevated erythrocyte sedimentation rate normalized too. On 09 September 2014 laboratory follow-up showed that all parameters were within normal ranges (Table 1.).
The patient first indicated improvements in breathing symptoms one month after diet onset. Thereafter symptoms improved gradually and completely disappeared within six months. Despite being without antihypertensive medication her blood pressure remained normal. Along with this, she reported significant improvement in physical well-being and quality of life. Although she reported that the diet was difficult to follow she was able to maintain it in the long term because she was highly motivated. In September 2014 she was able to return to work. Currently, she is on the paleolithic ketogenic diet for 10 months. She reported no side effects and is free of disease symptoms.
The patient gave written informed consent for writing this case study.
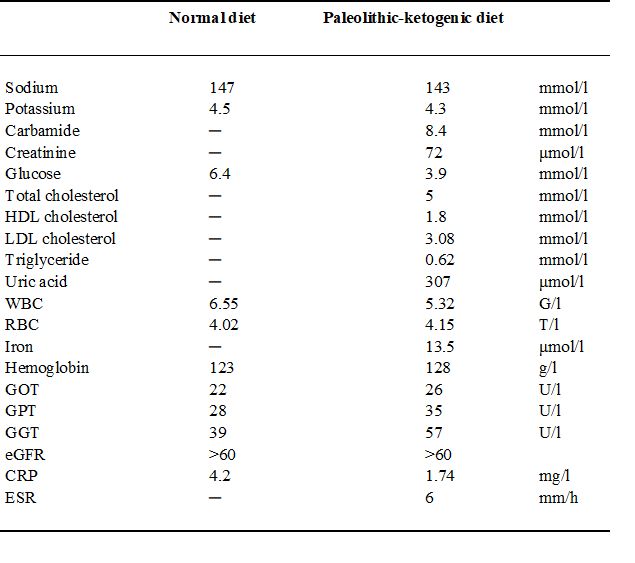
Table 1.
Laboratory data shortly before establishing diagnosis on a normal diet with seven medicines (on 29 November 2013) and at 9 months after diet initiation, on the paleolithic-ketogenic diet without medicines (on 09 September 2014). Note that all parameters fall in the normal range while on the paleolithic ketogenic diet. Dash indicates that a given parameter was not measured.
Abbreviations: WBC - white blood cell count, RBC - red blood cell count, eGFR - estimated glomerular filtration rate, HDL - high-density lipoprotein, LDL – low-density lipoprotein, CRP - C-reactive protein, ESR - erythrocyte sedimentation rate
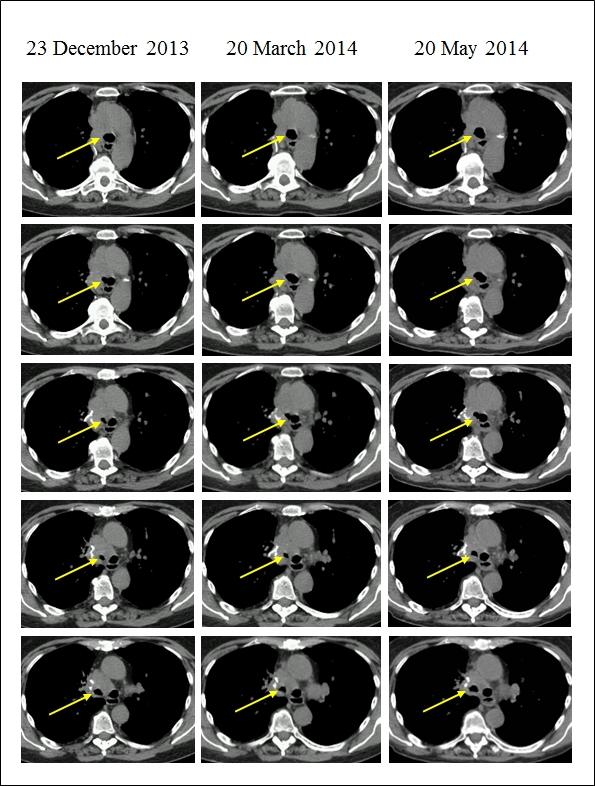
Five consecutive CT slices of the tracheal bifurcation and the main bronchi at three follow-up scans. Note the decreasing trend in the obstruction of the right main bronchus as indicated by increasing diameter of the lumen (arrow) across consecutive CT scans from 23 December 2013 to 20 May 2014. Also, note the decrease in subcutaneous fat as the patient lost weight.
Discussion
To our knowledge, this is the first report of successful treatment of a pulmonary tumor with a low carbohydrate diet. In the literature, there is only a single study reporting long-term survival with the classical ketogenic used in the case of two children with a malignant brain tumor (Nebeling et al., 1995). Short term positive result with the classical ketogenic diet was reported in a case of glioblastoma multiforme (Zuccoli et al., 2010) and in a mixed cohort of cancer patients in two systematic studies (Schmidt et al., 2011; Fine et al., 2012). In two recent studies of glioblastoma patients, the ketogenic diet proved to be safe, however, clinical benefit remains a question (Rieger et al., 2014; Champ et al., 2014).
Lung cancer is a leading cause of cancer death and bronchial tumors constitute 1-2 % of all pulmonary malignancies (Chughtai et al., 1997). Treatment options include surgery, chemotherapy, and radiotherapy. In localized tumors surgery, if possible, prolongs survival. However, chemotherapy and radiotherapy provide much less benefit. Overall survival for lung and bronchial tumors remains low with a five-year survival of 17 % (Ridge et al., 2013). Recent advances in molecular testing methods did neither substantially change the outlook of patients with lung cancer.
In experimental models, the ketogenic diet has been shown to inhibit the growth of tumors (Seyfried et al., 2011), and glucose level in mice was shown to proportionally relate to tumor growth (Seyfried, 2003). As a parallel in humans, high blood glucose levels predicted shorter survival in two studies of brain cancer patients (McGirt et al., 2008; Derr et al., 2009). According to Seyfried cancer can be viewed as a metabolic rather than a genetic disease and therefore tumor growth may be controlled by diet (Seyfried, 2012). Such a view is compatible with the original theory of Warburg postulating that tumor growth is driven by insufficient cellular respiration caused by damage to the mitochondria (Warburg, 1956). Due to this metabolic failure tumor cells largely depend on glucose for energy while incapable of using ketones.
The paleolithic ketogenic diet used in our patient not only induces ketosis but restricts foods not available in pre-agricultural times thus humans are not evolutionary adapted to. The paleolithic ketogenic diet used in our patient and in two cases reported previously (Clemens et al., 2013; Tóth and Clemens, 2014) closely resembles the meat-fat-based diet originally proposed by gastroenterologist Voegtlin (1975). Voegtlin (1975) and later Eaton and Conner (1985) as well as Cordain (2002) and Lindeberg (2009) suggested that western-type diet predominated by neolithic foods contribute to the development of degenerative diseases including cancer. In intervention studies, the paleolithic diet has been shown to provide metabolic advantages in patients with metabolic syndrome and type 2 diabetes (Jönsson et al., 2009; Frassetto et al., 2009; Mellberg et al., 2014). The classical form of the ketogenic diet has long been used in epilepsy (Sinha and Kossoff, 2005). A few reports extend the advantages of ketogenic diets to systemic diseases (Westman et al., 2007; Pérez-Guisado, 2007; Gedgaudas, 2011).
Here we report a case of recurrent bronchial cancer where we successfully applied the paleolithic ketogenic diet. Concurrent with the diet the patient received four cycles of chemotherapy but refused further cycles because of side effects. Thereafter she was treated with the paleolithic ketogenic diet only for six months.
Prognosis is poor for patients with recurrent lung cancer and for those not able to have surgery. Given that chemotherapy provides little clinical benefit we believe that in our patient positive results may be due to diet therapy rather than chemotherapy.
The patient continuously improved while on the diet. Along with this consecutive imaging data showed regression of the tumor. The patient reported constant ketosis as indicated by self-measurement by urinary ketone strips as well as urinary laboratory tests. Accordingly, the patient consistently showed low glucose levels on laboratory blood tests indicating strict adherence to the diet. At diet onset, she was able to discontinue her seven medications she was prescribed before. Her laboratory parameters including lipid profile and uric acid remained in the normal range while on the diet. During the four months of chemotherapy, blood cell counts decreased but increased to normal levels when stopping the chemotherapy.
Despite discontinuing antihypertensive medication at diet onset, the patient reported having consistently normal blood pressure. Contrary to the usual belief that patients without a gall bladder are at risk when put on a high-fat diet, our patient did neither report gastrointestinal symptoms.
At the time of writing this publication the patient is on the paleolithic ketogenic diet for 10 months, reports neither side effects nor lung disease symptoms. Her fatigue resolved and she was able to return to work in September 2014. She is reporting an excellent quality of life.
Cancer experts are generally skeptical regarding dietary approaches. Certainly, this is due to the relatively few cases with long-term positive outcomes in the literature. However, another reason may be that the ketogenic diet in its classical form, is indeed of limited effectiveness. We suggest that the paleolithic ketogenic diet may be advantageous over the classical form of the ketogenic diet. The paleolithic ketogenic diet may be remedial by multiple mechanisms such as inducing ketosis as well as restricting “non-paleolithic” foods that may boost cancer growth (Cordain, 2002).
References
Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, Wortman JA, Yancy WS, Phinney SD. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007
Aug;86(2):276-84.
Pérez-Guisado J. Arguments In Favor Of Ketogenic Diets . The Internet Journal of Nutrition and Wellness. 2007 Volume 4 Number 2
Kossoff EH. More fat and fewer seizures: dietary therapies for epilepsy. Lancet Neurol. 2004 Jul;3(7):415-20.
Ely JTA. Glycemic modulation of tumor tolerance. J Orthomolecular Med. 1996;11:23-34.
Klement RJ, Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond). 2011 Oct 26;8:75.
Seyfried TN, Kiebish MA, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. Metabolic management of brain cancer. Biochim Biophys Acta. 2011 Jun;1807(6):577-94.
Seyfried TN. Cancer as a Metabolic Disease: On the Origin, Management, and Prevention of Cancer. 2012 Hoboken, New Jersey, Wiley
Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995 Apr;14(2):202-8.
Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, Seyfried TN. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr Metab (Lond). 2010 Apr 22;7:33.
Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr Metab (Lond). 2011 Jul 27;8(1):54.
Fine EJ, Segal-Isaacson CJ, Feinman RD, Herszkopf S, Romano MC, Tomuta N, Bontempo AF, Negassa A, Sparano JA. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012 Oct;28(10):1028-35.
Rieger J, Bähr O, Maurer GD, Hattingen E, Franz K, Brucker D, Walenta S, Kämmerer U, Coy JF, Weller M, Steinbach JP. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014 Jun;44(6):1843-52.
Fine EJ, Segal-Isaacson CJ, Feinman RD, Sparano JA. Carbohydrate restriction in patients with advanced cancer: a protocol to assess safety and feasibility with an accompanying hypothesis. Community Oncology January 2008
Voegtlin WL. The stone age diet: based on in-depth studies of human ecology and the diet of man. New York: Vantage Press; 1975.
Cordain L. The paleo diet: lose weight and get healthy by eating the food you were designed to eat. New York: Wiley; 2002.
Clemens Z, Kelemen A, Fogarasi A, Tóth C. Childhood absence epilepsy successfully treated with the paleolithic ketogenic diet. Neurol Ther. 2:71–76. 2013
Tóth C, Clemens Z. Type 1 diabetes mellitus successfully managed with the paleolithic ketogenic diet. Int J Case Rep Images 2014;5(10):699–703.
Champ CE, Palmer JD, Volek JS, Werner-Wasik M, Andrews DW, Evans JJ, Glass J, Kim L, Shi W. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014 Mar;117(1):125-31.
Chughtai TS, Morin JE, Sheiner NM, Wilson JA, Mulder DS. Bronchial carcinoid--twenty years' experience defines a selective surgical approach. Surgery. 1997 Oct;122(4):801-8.
Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013 Jun;30(2):93-8.
Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer. 2003 Oct 6;89(7):1375-82.
McGirt MJ, Chaichana KL, Gathinji M, Attenello F, Than K, Ruiz AJ, Olivi A, Quiñones-Hinojosa A. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008 Aug;63(2):286-91.
Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009 Mar 1;27(7):1082-6.
Warburg O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309-14.
Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985 Jan 31;312(5):283-9.
Lindeberg S. Food and western disease: health and nutrition from an evolutionary perspective.
Chichester: Wiley-Blackwell; 2009.
Jönsson T, Granfeldt Y, Ahrén B, Branell UC, Pålsson G, Hansson A, Söderström M, Lindeberg S. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009 Jul 16;8:35.
Frassetto LA, Schloetter M, Mietus-Synder M, Morris RC Jr, Sebastian A. Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. Eur J Clin Nutr. 2009 Aug;63(8):947-55.
Mellberg C, Sandberg S, Ryberg M, Eriksson M, Brage S, Larsson C, Olsson T, Lindahl B. Long-term effects of a Palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014 Mar;68(3):350-7.
Sinha SR, Kossoff EH. The ketogenic diet. Neurologist. 2005 May;11(3):161-70.
Gedgaudas NT. Primal body, primal mind: beyond the paleo diet for total health and a longer life. Healing Arts Press, Rochester, Vermont, Toronto, Canada, 2011
 Rehabilitáció csak online elérhető
Rehabilitáció csak online elérhető
 E-mail: paleomedicina@gmail.com
E-mail: paleomedicina@gmail.com

