VITAMIN C AND DISEASE: INSIGHTS FROM THE EVOLUTIONARY PERSPECTIVE
Abstract
The role of vitamin C at the physiological and cellular levels is indisputable. In line with this, blood level of vitamin C is inversely related to disease parameters such as risk of cancer, cardiovascular disease and mortality in prospective cohort and correlational studies. At the same time, adequately powered clinical intervention studies consistently provide no evidence for a beneficial effect of supplementing vitamin C. Here we provide a framework to resolve this apparent conflict. Besides providing an overview of the widely-known facts regarding vitamin C, we review evidence that are of potential relevance but are seldomly mentioned in the context of vitamin C. We invoke the glucose-ascorbate antagonism (GAA) theory which predicts that as a consequence of their molecular similarity glucose hinders the entry of vitamin C into cells. Integrating data coming from research at the cellular level, those from clinical, anthropological and dietary studies, in the present hypothesis paper we propose an evolutionary framework which may synthesize currently available data in the relation of vitamin C and disease. We put forward that instead of taking vitamin C as a supplement, an evolutionary adapted human diet based on meat, fat and offal would provide enough vitamin C to cover physiological needs and to ward off diseases associated with vitamin C deficiency.
1. Introduction
Vitamin C is one of the most widely taken nutritional supplements [1,2]. Health professionals as well as laymen attribute a number of health benefits to vitamin C such as boosting the immune system or preventing the common cold and cancer. Whilst the role of vitamin C in physiologic processes is well-established, there is little or no high-grade evidence supporting that taking vitamin C as a supplement is indeed beneficial. We will summarize existing research data with a special emphasis on aspects that are seldomly mentioned in the context of vitamin C. As such, we invoke the glucose-ascorbate antagonism (GAA) theory, which proposes that availability of vitamin C for cells is determined by glucose concentration [3]. We review evidence from cellular physiology as well as dietary studies carried out in Inuit people to support the GAA theory and the view that the source of vitamin C as well as composition of diet is crucial for optimal vitamin C supply. In this review we propose a concept which may be suitable for synthesizing data coming from diverse sources and/or that are apparently conflicting. The frames of the hypothesis are formed by a major, albeit often neglected principal: evolution. We put forward that apparent controversies regarding vitamin C can be readily resolved by an evolutionary approach.
2. Biosynthesis and biological significance of vitamin C
In most mammals vitamin C is produced from glucose in the liver. Species that are unable to synthesize this nutrient rely on dietary uptake from its food sources [4]. Vitamin C, in synergy with vitamin E, is known to have a role in reducing lipid peroxidation [5]. Vitamin C also acts as a co-factor in at least eight enzymatic reactions including those involved in the synthesis of collagen and carnitine [6].
Vitamin C exists in two redox states: ascorbic acid (AA), and its oxidized form, dehydroascorbic acid (DHAA). Most actions of vitamin C can be attributed to AA which acts as a reducing agent donating electrons to various reactions [7]. The oxidised form of vitamin C is then recycled back to AA. Both AA and DHAA are absorbed in the small intestine [7]. Absorption is almost complete at doses <200 mg but the degree of absorption decreases as intake increases [8]. Uptake of AA from the intestine relies on an active transport mechanism of sodium dependent vitamin C transporters (SVCT) while uptake of DHAA relies on facilitative diffusion by facilitative glucose transporters (GLUT) [9]. Within the enterocytes, absorbed DHAA is converted to AA thereby producing low intracellular DHAA concentration which by a gradient facilitates further DHAA uptake. DHAA may be taken up from the blood by several cell types that reduce it to AA. DHAA can be filtered from the plasma by renal corpuscules and then reabsorbed in the renal tubules for subsequent reduction [10]. Some organs accumulate vitamin C (AA) 10-50-fold higher than the blood level of it. These include tissues of high metabolic activity such as adrenal glands, thymus, eye lens, retina, brain, pancreas, kidney, lymph nodes and lymphocytes [11].
AA cannot penetrate the blood brain barrier thus vitamin C is taken up in its oxidised form DHAA which is then reduced back to AA for retaining it within brain cells [12]. A similar transport mechanism exists for vitamin C entering the mitochondria [9]. AA is accumulated within the mitochondria where it has a role to scavange free radicals abundantly produced by mitochondrial function [13]. Several pathological conditions have been shown to be associated with impaired redox cycling of vitamin C. For example, conditions associated with inflammation such as diabetes, trauma, surgery, sepsis and wound healing are also known to be characterized by decreased AA concentrations along with elevated DHAA concentrations in both plasma and leucocytes [10]. This is believed to be due to increased level of oxidants such as hydroxyl radicals, peroxyl radicals and superoxide anion requiring more AA than could be regenerated from DHAA [10].
3. Shortage and overdose of vitamin C
Shortage of vitamin C causes scurvy, presenting with symptoms including malaise, gum disease, poor wound healing, shortness of breath and bone pain. Scurvy occurred frequently among salesmen in medieval times. In current western societies it may occur in subjects with alcoholism where it typically presents along with deficiencies of other vitamins [14]. Oral megadose (>1000 mg) of vitamin C may cause diarrhea, nausea, abdominal bloating and heatburn [4]. There is evidence that vitamin C supplementation increases the risk of forming kidney stones [4]. Megadoses of vitamin C may lead to the development of vitamin B12 deficiency [4], a phenomenon explained by redox reactions of AA converting vitamin B12 into a biologically inactive analogue [6]. As an additional side effect, in glucose-6-phosphate dehydrogenase deficiency AA has been shown to have a hemolytic effect [6]. Intrevenous megadose of vitamin C may result in diarrhea, nausea, headache, decreased appetite and fatigue [16].
4. Evolutionary perspective
Vitamin C is an essential nutrient for humans who are unable to synthesize and thus have to obtain it from its dietary sources. Loss of the ability to synthesize vitamin C is not unique among mammals: e.g. guinea pigs, monkeys and apes also lack this ability. Non-synthesizing species, including humans, lack the L-gulonolactone oxidase (GULO) enzyme which is required in the last enzymatic step of synthesis of vitamin C from glucose [4]. In the genome of humans and that of the anthropoid primates a non-functional gene is present instead of the GULO gene. Loss of the ability to synthesize vitamin C in primates is believed to have occurred about 60 million years ago at the time of the split of the two primate suborders: Strepsirrhini and Haplorhini [17]. Strepsirrhine primates including lemurs, lorises, and galagos are able to produce vitamin C while haplorhine primates such as tarsiers, monkeys and apes obtain it from dietary sources.
In the medical literature losing the ability to synthesize vitamin C in the course of evolution is usually interpreted as an imperfection leaving our health vulnerable. However with an evolutionary attitude such an ”imperfection” is inconceivable given that species that are currently living represent the highest level of adaptation to their environment. Non-human species lacking the GULO gene are not regarded imperfect either. It would be hard to believe that the Homo genus would have been so successful in terms of survival and spatial spread with a deteriorating genetic mutation. We assume that in the ancestral environment, where our hominoid predecessors lived for 2.6 million years, loss of internal synthesis of vitamin C was not disadvantegous. As one possible explanation, Ames et al. [18] proposed that during the primate evolution uric acid might have taken over the antioxidant function of vitamin C, a hypothesis based on the striking paralellism between the inability to break down uric acid and the loss of the ability of vitamin C synthesis in primates [19].
5. Dietary sources of vitamin C
Recommended Dietary Allowances (RDA) of vitamin C, as suggested by the Institute of Medicine, is 90 mg for males and 75 for females. According to the NHANES survey between 2003 and 2004, 7% was vitamin C deficient (serum concentration <11.4 μmol/L) in an US population where 37% of men and 47% of women was taking vitamin C supplements [20].
The estimated average requirement (EAR) is set at 75 mg/d on the basis that it will lead to 80% saturation of neutrophil AA concentrations without substantial urinary excretion, thereby maximizing antioxidant effects. The RDA is derived from the EAR by assuming a coefficient of variation (CV) of 10% in nutritional needs and adding twice the CV to the EAR to yield an RDA that presumably covers the needs of 97-98% of the population [21]. Failure or inconclusiveness of intervention studies with vitamin C may be due to the fact that vitamin C in these studies was given to people who had already been meeting or nearly meeting their requirement as defined by the RDA.
Current dietary recommendations concentrate on plant sources of vitamin C. The WHO, for example, recommend a minimum of 400g of fruit and vegetable per day also in an attempt to ensure other micronutrients [22]. The belief that plant sources are ideal for vitamin C supply roots back to James Lind who in 1747 discovered that scurvy, a devasting disease of English sailors, can be prevented and reversed by fresh fruits. In fact, several lines of evidence indicate that people may remain free of scurvy on diets lacking fruits and vegetables.
In hunter-gatherer societies organ meats and especially liver, marrow and brain are highly favoured. These latter organs, as also described above, accumulate high level of vitamin C [23,24] and are rich in other vitamins too [25]. At odds with dietary recommendations in the western world, the majority of hunter gatherer societies are subsisting on meat based diets yet remain free of scurvy [26,27,28]. In the study of Cordain et al. [26] 168 of the 229 hunter gatherer societies subsisted on diets containing at least 55% hunted and/or fished food. In fact the ratio of animal food may be even higher given that hunther gatherers also eat insects, invertebrates and other small animals that are collected by gathering [26]. If both plant and animal foods are available, hunther gatherers clearly prefer the latter [26,27].
Traditionally living arctic people represent extreme examples of animal food reliance. Among the Inuit scurvy was not observed until the 20th century [23]. Studies show that the traditional diets of northern populations, including the Inuit diet, provide enough amount of vitamin C. Fediuk [23], for example, reported an average intake of 38 mg of vitamin C/day from meat and organs. However, on special occasions such as after successful whale hunts intake may reach 340 mg/day [23]. Animal parts with highest vitamin C content include ”muktuk”, epidermis of the beluga whale (containing 36 mg/100 mg of vitamin C) and the liver (containing 24 mg/100 mg of vitamin C) as well as the brain (containing 15 mg/100 mg of vitamin C) of land and sea mammals [23,24]. In the 1970th, in the study of Geraci and Smith [29] daily intake of vitamin C in a hunting Inuit community was in the range 10-120 mg, much higher than found earlier in a national survey of Canada assessing Inuit populations in larger settlements with transitional cultures [29]. Westernalization of native societies brought substantial changes in life style along with a decline in the access to traditional foods. In parallel, an increasing portion of the Inuit people became at risk for scurvy [23].
Several examples show that not only native people may subsist on meat-fat based diets. It is known that arctic travellers of European descent living on canned Western type food were frequently affected by scurvy but this was not the case in those who had access to traditional foods [25]. The arctic explorer Vilhjalmur Steffanson, for example, lived on the Inuit diet for nine years and remained completely healthy. In 1930 an experiment was set up in which Steffanson along with a fellow explorer lived on an exclusive meat diet for one year [30]. No sign of vitamin deficiency, including scurvy, was noticed despite of the absence of vitamin supplementation. Voegtlin, first proponent of the human evolutionary diet, put forward that the diet humans are evolutionary adapted to is based on animal fat, meat and offal, and is of full nutritional value. Along with this, he argued against the usefulness of supplementing vitamin C [25]. We would like to emphasize that those hunter gatherers subsisting on diets containing larger amounts of fruits and vegetables, may also have access to enough vitamin C. This may be due to the combined sources of animal and plant vitamin C along with a carbohydrate intake being still much lower than on an average Western type diet. Anyhow, the Kitavan islanders for example, who are known to consume much carbohydrate as compared with other indigenous people, exhibit low blood glucose levels (on average 3.5 mmol/l in the young population) [31].
The authors of the present paper are rehabilitating patients with chronic diseases by using a diet we refer to as the paleolithic ketogenic diet [32-36]. This is an animal fat-meat based diet which is close to the diet originally proposed by Voegtlin [25]. The paleolithic ketogenic diet excludes foods that were not available for preagricultural humans such as cereal grains, milk and dairy, vegetable oils, nightshades, legumes, refined sugars and foods with additives. It also excludes foods that may be included in the popular versions of the paleolithic diet such as oilseeds, coconut, coconut oil, artificial sweeteners, vitamin as well as other supplements. The paleolithic ketogenic diet also differs from the popular paleolithic diet in that it restricts vegetables and fruits to an amount of <30% (by weight) and thus ensures ketosis. The suggested fat:protein macronutrient ration is 2:1 (in grams). We encourage red and fat meats and the regular intake of organ meats. Like other proponents of the paleolithic diet [25,26] we advise patients against taking vitamin C supplements. Our clinical experience, also shown by the example of our published cases [32-36], indicate that neither scurvy nor other nutritional deficiency emerge in the absence of vitamin C supplementation while adhering to the paleolithic ketogenic diet. As an important distinction, this is not the case with the classical form of the ketogenic diet where scurvy may occur [37] likely due to limited intake of both animal and plant sources of vitamin C.
Another potential benefit of animal derived vitamin C pertains to cooking. It is widely known that vitamin C content of foods degrade at higher temperatures. However, cooking-related loss of vitamin C seems to be smaller in animal-derived foods as compared with plant-derived ones as assessed both by the analysis of the Inuit diet [29,23] and by comparison of food items in publicly available food databases. For example, according to the USDA's National Nutrient Database for Standard Reference in the raw spinach there is 28.1 mg/100 g of vitamin C while cooked spinach contains only 9.8 mg/100 g of it. At the same time, raw and cooked forms of pork liver contain comparable amounts of vitamin C: while the former contains 25.3 mg/100 g the latter contains 23.6 mg/100 g of vitamin C.
6. Vitamin C and disease
In the following we review data from those clinical studies representing the highest grade evidence available and have a hard clinical endpoint. In such an attempt we have searched pubmed and google scholar databases for studies that include terms ”vitamin C” or ”ascorbic acid” in combination with ”mortality”, "cancer”, ”cardiovascular disease”, ”stroke”, ”hypertension” and ”common cold”. We also searched for ”intravenous vitamin C therapy”. We then reviewed search results for each of the above condition to select those studies that (1) include the most subjects/patients, (2) have a sound methodology and (3) and assess a hard endpoint/clinically meaningful variable. Parameters which were regarded as hard clinical endpoint include: bivariate variables (such as death or the occurrence of specific events including stroke or cardiovascular events, and having a diagnosis of a specific disease), survival time, incidence of common cold, and blood pressure itself in hypertension. We disregarded studies that rely on soft clinical endpoints of disease e.g. cholesterol level in cardiovascular disease, the role of which in cardiovascular disease is debated. Prospective cohort and correlational studies are presented separately from intervention studies. For prospective and correlational studies we only included studies that assessed blood level of vitamin C and excluded those that rely on estimated dietary intake of vitamin C. Reasons for this include probable inadequacies in estimation of the vitamin C content of foods and the low/variable correlation between dietary intake and the blood level of vitamin C [38]. The low measured correlation between dietary and blood vitamin C in these studies is due to fact that blood vitamin C not only depend on intake but on other factors including differences in relative partitioning between plasma and intracellular concentration as well as differences in excretion and oxidation rates [6]. From our point of view it is of upmost importance that dietary studies assessing vitamin C intake, either epidemiological or interventional studies, limited the estimation of vitamin C to the plant sources while neglecting the animal sources of it. The reason for selecting the conditions of common cold, cardiovascular disease and cancer in the present manuscript lies in the fact that large studies of vitamin C (either prospective or randomized controlled studies) are available for these conditions only. Out of the three conditions, cancer will be in the focus, given the predominance of studies assessing cancer.
6.1. Prospective cohort and correlational studies: blood level of vitamin C and disease
For an overview of prospective cohort and correlational studies of blood level of vitamin C and disease including mortality, cancer, cardiovascular disease, stroke and hypertension see Table 1.
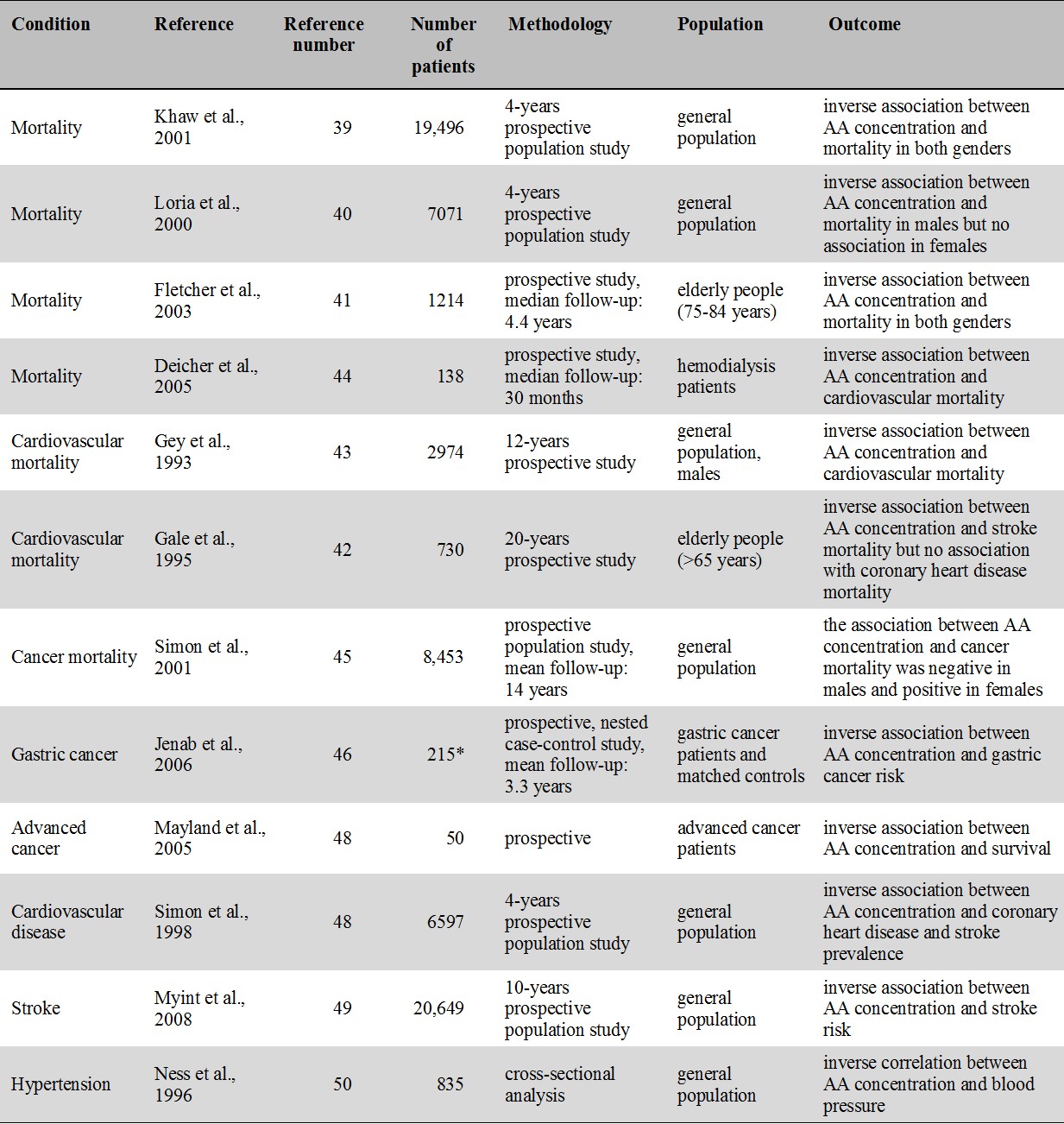
Table 1.
Major prospective cohort and correlational studies of vitamin C and disease
* number of patients in the gastric cancer group
Note that all studies indicate an inverse association between blood concentration of AA and mortality/morbidity.
6.1.1. Mortality
In the EPIC-Norfolk prospective population study, plasma concentration of AA was inversely related to mortality from all-causes as well as from cardiovascular disease and ischaemic heart disease. Mortality risk in the top quintile was half the risk in the lowest quintile [39]. A study based on an NHANES II. database found an inverse relation between dying from any cause and low AA in men but there was no association in women [40]. In one study with elderly, AA concentration was shown to be inversely associated with subsequent mortality in both males and females [41] while in another study AA concentration was inversely associated with mortality from stroke but not from coronary heart disease [42]. Male cardiovascular mortality in the Basel Prospective Study was also inversely associated with plasma level of vitamin C [43]. In hemodialysis patients, low level of vitamin C was shown to be a risk factor for cardiovascular morbidity and mortality which was explained by vitamin C ameliorating vascular dysfunction generally seen in patients with renal failure [44].
5.1.2. Cancer
In the EPIC-Norfolk study, vitamin C level was inversely related to cancer mortality in males but not in females [40]. In an NHANES II. study, cancer risk was inversely related to AA in males [45]. Unexpectedly, in females high level of AA was found to be associated with increased cancer risk [45]. A case control study showed increased risk for gastric cancer for those with low plasma level of vitamin C [46] but no association was found for dietary vitamin C intake [46]. In hospice patients low plasma concentrations were associated with shorter survival [47].
6.1.3. Cardiovascular disease
In an NHANES II. study, serum AA was inversely related with the prevelance of coronary heart disease and stroke [48]. In several studies, including the Epic-Norfolk prospective population study, low plasma concentration of vitamin C was associated with increased stroke risk [49]. In cross-sectional studies, blood pressure in middle aged and elderly [50] as well as in young adults [51] was found to be inversely associated with blood AA.
6.2. Intervention studies of vitamin C
6.2.1. Oral supplementation of vitamin C
For an overview of the oral vitamin C intervention studies related to mortality, common cold, cardiovascular disease and high blood pressure see Table 2. Given the high number of intervention studies with vitamin C available in the literature, in the present review we concentrate on RCTs and metaanalyses.
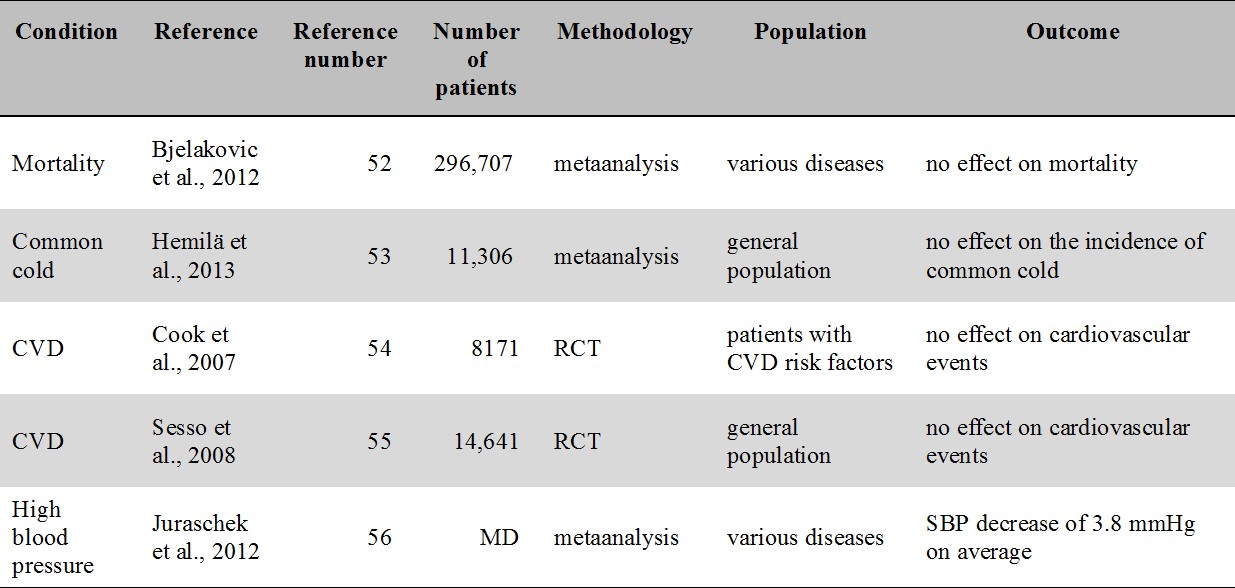
Table 2.
Major metaanalyses and RCTs of oral vitamin C supplementation related to mortality, common cold, cardiovascular disease and hypertension. No study report a clinically meaningful benefit.
CVD: cardiovascular disease; MD: missing data; SBP: systolic blood pressure
6.2.1.1. Mortality
A recent Cochrane review examining mortality as an endpoint found that supplementation of antioxidants including vitamin C have no effect on overall mortality [52].
6.2.1.2. Common cold
The latest Cochrane review [53] concluded that vitamin C supplementation has no effect on the incidence of common cold. However, a modest reduction of sympoms was consistently found in the reviewed studies [53].
6.2.1.3. Cardiovascular disease
The Women's Antioxidant Cardiovascular Study, a randomized controlled study (RCT), found no effect of vitamin C supplementation in the secondary prevention of cardiovascular disease [54]. Another RCT, the Physicians' Health Study II., did neither find vitamin C to be beneficial in the prevention of cardiovascular events [55]. In regards to high blood pressure, a metaanalysis of twenty-nine trials of blood pressure indicated a statistically significant yet clinically not meaningful decrease of 3.8 mmHg in systolic blood pressure [56].
6.2.1.4. Cancer
The idea that vitamin C may be beneficial in cancer treatment stems from Linus Pauling who argued that high doses of vitamin C (10 g/day) given intravenously may be useful in the treatment of cancer [57]. This assumption was based on a study of 100 patients with advanced cancer who as compared to historical controls survived three to four times longer [57]. The study was later criticized because of the absence of an appropriate control group. The study was repeated with improved methodology and the authors still reported survival benefit in the vitamin C group [58,59]. However, subsequent prospective controlled studies that were carried out at the Mayo clinic were unable to repeat results. These studies, however, used oral administration of vitamin C rather than intravenous administration [60,61]. Unlike the original study [57], Creagan et al. [60] included patients who previously received chemotherapy while the study by Moertel et al. [61] included cancer patients with no prior chemotherapy.
Prospective RCTs, like the Physicians’ Health Study II., did not find vitamin C supplementation to be effective in the prevention of total cancers, prostate and in other solid tumors [62]. No effect of vitamin C supplementation on cancer incidence and mortality was seen in another randomized trial, the Women's Antioxidant Cardiovascular Study [63]. A metaanalysis by Coulter et al. [64] also concluded that vitamin C provide no prevention of cancer.
For an overview of the oral vitamin C intervention studies related to cancer see Table 3.
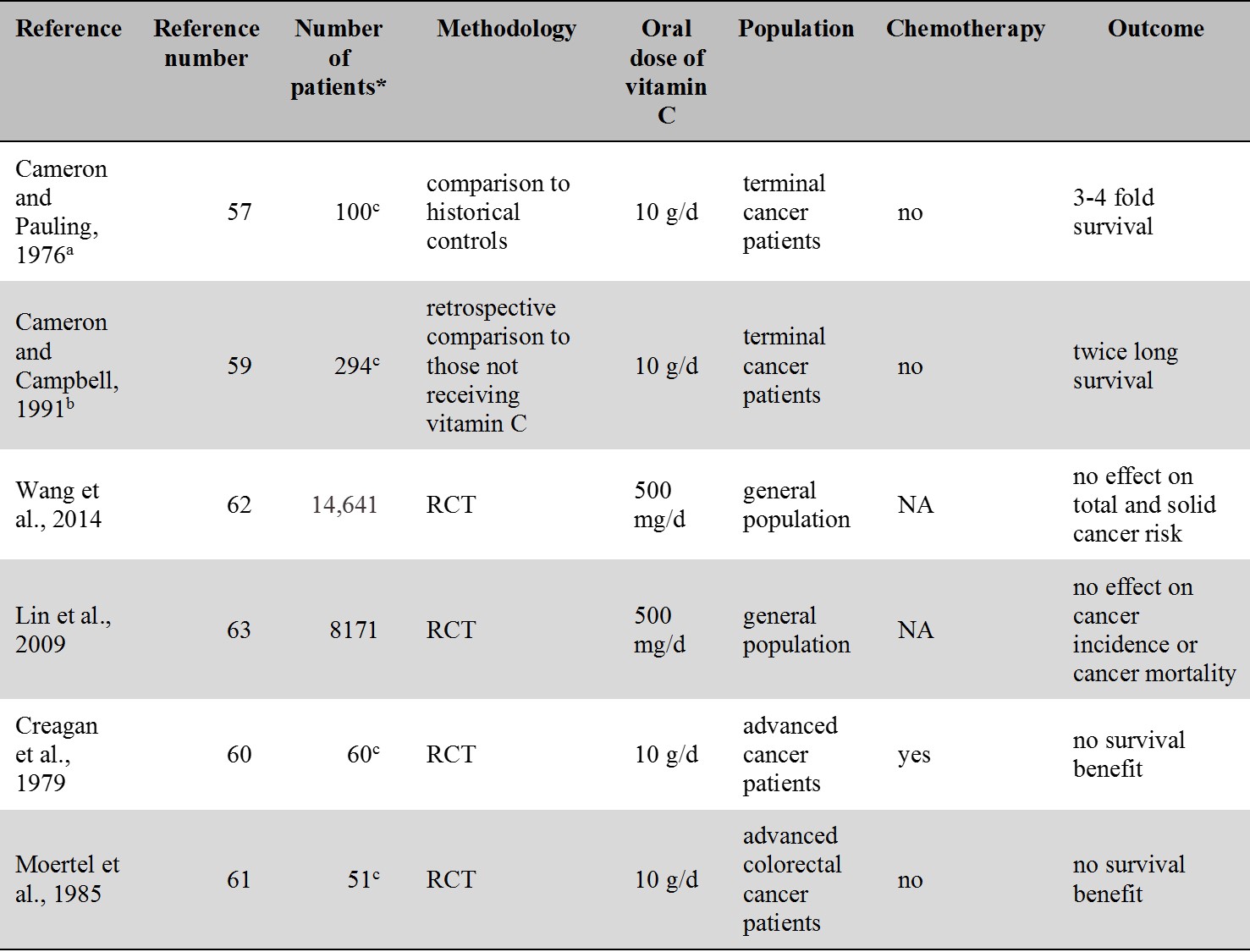
Table 3.
Cancer-related intervention studies of oral vitamin C.
a patients received IVC therapy for 10 days followed by oral vitamin C
b besides oral vitamin C some patients received IVC therapy too
c number of patients in the vitamin C group
* Number of patients treated with vitamin C
RCT: randomised controlled trial; NA: not applicable
6.2.2. High dose intravenous vitamin C (IVC) therapy in cancer
Vitamin C is known to poorly absorb when administered orally at higher doses: immune cells saturate at 100 mg daily, and renal excretion of AA increases above this dose [8]. Therefore, it was claimed that blood concentrations of vitamin C in the Mayo Clinic studies [60,61] might not have reached a concentration producing the cytotoxic effect to tumor cells seen in in vitro studies [65]. With intravenous administration, the tightly regulated absorption and transport of vitamin C is bypassed and therefore result in a much (30- to 70-fold) higher than physiological blood level of vitamin C [66]. Cytotoxicity of AA to cancer cells was based on in vitro studies which suggested that AA in concentrations higher than physiological in the extracellular space act as a pro-oxidant and through the formation hydrogen peroxide kill cancer cells [67]. However, in a recent study the cytotoxic effect was abolished at physiological concentrations of iron which prevented the accumulation of hydrogen peroxide [68]. It was suggested that previous studies disregarding in vivo concentration of iron had significantly overestimated the anticancer effect of AA [68].
Currently, no more than a single RCT is available that assessed the effect of IVC therapy in cancer patients [69]. This indicated a reduction of chemotherapy induced side effects in ovarian cancer patients but no significant effect in terms of survival was found [69]. In an uncontrolled study of metastatic pancreatic cancer patients, Monti et al. [70] reported minor decrease in the size of the tumor without evidence of prolonged survival. In another study, of the 16 patients with solid tumors no one experienced an objective tumor response [71]. In a study by Mikirova et al. [72], decreased level of inflammatory markers was seen following IVC therapy but no positive effect was reported on disease progression and survival. Clearly, no antitumor effect was evident in phase I. clinical trials studies including studies by Welsh et al. [73] and Hoffer et al. [74]. Two other clinical group studies by Riordan [75] and Yeom et al. [76] did neither provide evidence for an anticancer effect. A more recent phase I-II. clinical study by Hoffer et al. [77] reported transient (lasting for 3-13 months) stable disease in 3 out of the 16 patients in the study. Actually all the existing group studies have failed to provide evidence for a benefit in hard clinical endpoints such as survival. At the same time, it is important to mention that except for the seminal study of Cameron and Pauling [57] all the above IVC studies included patients with prior or concurrent chemotherapy. Thus the possibility suggested by Creagan et al. [60] that cancer patients with no chemotherapy may benefit more from IVC therapy cannot be entirely excluded.
Currently only case reports are available describing survival benefit following IVC therapy [78,17]. Overall, from the available group studies it only seems that high dose IVC therapy is relatively safe, reduces chemotherapy induced side effects but provide no benefit in terms of hard clinical endpoints including survival in cancer patients also receiving chemotherapy.
For an overview of the IVC studies related to cancer see Table 4.
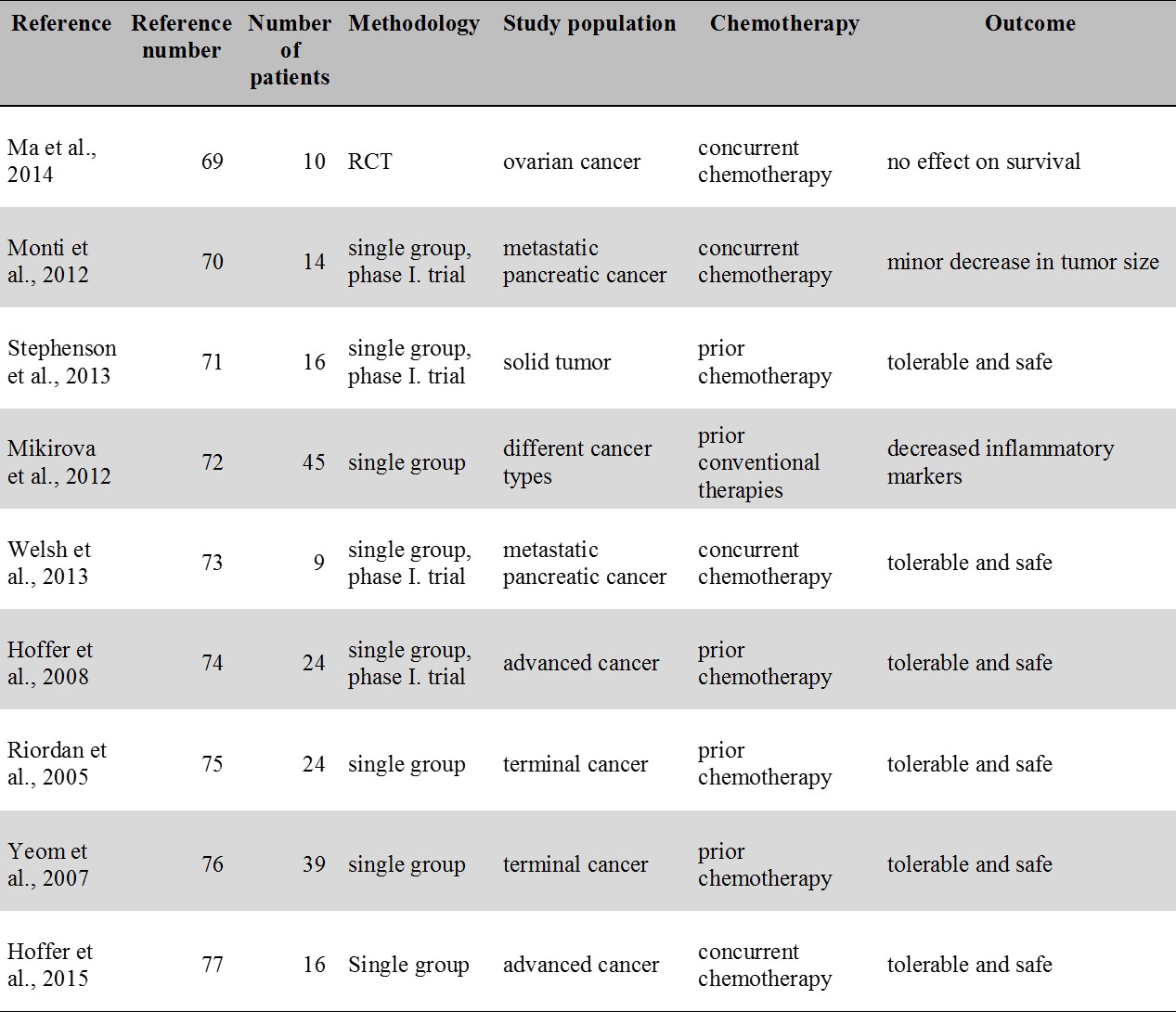
Table 4.
IVC therapy studies in cancer. Except for a small RCT (Ma et al. 2014) all studies contain only a single group. Each study included patients with prior or concurrent chemotherapy. No study reported an apparent benefit in terms of survival.
ICV: intravenous vitamin C; RCT: randomized controlled trial
6.3. Summary of the prospective cohort and intervention studies of vitamin C
Prospective cohort studies unequivocally found an inverse relation with the blood level of vitamin C for mortality, cancer and cardiovascular disease for each of the specific disease populations. Two prospective studies [40,45] when assessing males and females separately, reported the inverse association in males only. We assume that the absence of an inverse association in females may be due to differential habits of males and females in taking supplements. Although in the two studies it was not possible to retrieve data on supplement use, females are known to be more prone to take vitamin C supplements [20], and thus a higher serum level of AA in females may reflect the effect of supplementation which may not be preventive. Overall results from prospective studies are in sharp contrast with the intervention studies where none of the studies reported a clinically meaningful benefit as regards mortality, incidence of common cold, cardiovascular events, and prevention or treatment of cancer.
7. The glucose-ascorbate antogonism theory
The glucose-ascorbate antagonism (GAA) theory was first proposed by John Ely as early as the 1970s [3]. The theory postulates that given the structural similarity between glucose and vitamin C, the two molecules compete for same transport system to enter cells [10]. It has been shown that cellular uptake of both AA and DHAA may be competitively antagonized by elevated glucose levels. Specifically, AA uptake by the small intestine enterocytes was shown to be inhibited by elevated glucose concentration [9]. DHAA transport into cells was shown to be impaired by high blood glucose concentration in most cell types including adipocytes, erythrocytes, granulosa cells, neutrophils, osteoblasts and smooth muscle cells [10].
A potential limitation of the GAA theory pertains to the lack of in vivo studies in animals and in humans. Diabetes, however, can be regarded as a natural model to study interaction between blood glucose and vitamin C in vivo in humans. Studies with diabetic patients are in line with the GAA therory. Two studies in type 2 diabetes [79,80] indicated that in spite of similar dietary intake of vitamin C, patients have decreased levels of plasma AA as compared to normal controls. Furthermore, serum level of AA inversely correlated with glucose levels [80] and in another study with glycated hemoglobin of diabetic patients [81]. Diabetes is also known to be associated with impaired renal reabsorption of AA [82] which likely contributes to the low level of AA in patients.
Uptake of glucose and DHAA also share the feature of insulin-dependency on the GLUT4 glucose transporter primarily found in muscle and adipose tissue [83]. In type 1 diabetes, deficiency of insulin has been shown to impair DHAA uptake of lymphoblasts [84]. Such a decreased DHAA uptake, through impaired AA accumulation, may lead to compromized immune system function in type 1 diabetes patients [84]. The effect of glucose seems to be immediate on intracellular AA given that intravenous glucose administration results in a prompt decrease in the AA concentration of leukocytes [85]. Also consistently with the GAA theory, blood level of vitamin C is inversely related with obesity [86] a correlate of increased carbohydrate intake.
Controlling hyperglycemia has also been suggested as an adjunct to cancer therapy [87]. Hyperglycemia in cancer patients is known to be associated with reduced intracellular AA concentration. Such a decrease results in impaired actions of AA, including a decreased activity of the hexose monophosphate shunt, a patway important in optimal immune cell functioning [87]. In cardiovascular disease, the association of high blood glucose and low AA concentration is also consistent with the GAA mechanism, and may plausibly explain functional impairments such as lipid peroxidation and endothelial dysfunction which are known to contribute to the generation of atherosclerosis [87].
As an additional parallelism, the study by Johnstone et al. [88] compared the effect of two high protein diets: one with low (in fact ketogenic) and another with medium carbohydrate content. To the surprise of the investigators, higher concentration of blood vitamin C was found on the low carbohydrate diet. Yet, the difference was explained by other factors.
AA is known to have a role in scavenging reactive oxygen species within the mitochondria. Reactive oxygen species within the mitochondria have been suggested to have a role in the development of degenerative disorders including cancer [89]. Given the fact that carbohydrate based Western type nutrition is associated with increased production of reactive oxygen species in the mitochondria [89], it is plausible to speculate that the need for AA would be decreased when on a low carbohydrate diet.
In human brush border membrane vesicles evaluated ex vivo, AA uptake is competitively antagonized by glucose [9] coming abundantly from carbohydrate based diets may explain why according to a metaanalysis dietary intake and plasma level of AA were only moderately correlated [38]. Importantly, estimation of AA intake in this study relied on plant sources only. Negligation of animal sources of vitamin C is typical in the case of the other dietary studies too. This fact together with the loose association between the intake estimate and blood level indicate that plants are not ideal sources of vitamin C. Additionally, flavonoids abundantly found in fruits and vegetables were also shown to inhibit vitamin C uptake by enterocytes [90]. We are not aware of studies assessing the relation between animal sources of vitamin C and blood level of it. The striking dissociation between epidemiological and intervention studies, as revealed in this review for mortality, cardiovascular disease and cancer, may be regarded as an indication that vitamin C taken as a supplement may not be an optimal way to obtain this nutrient.
Chronic diseases such as obesity, diabetes, neurodegenerative diseases, cancer and cardiovascular diseases being associated by the cluster of carbohydrate overconsumption [91], mitochondrial dysfunction [92], increased production of reactive oxygen species [92] and decreased blood level of vitamin C also point to the GAA as a common mechanism beyond these pathologic conditions. It can be speculated that increased dietary intake of carbohydrates and resulting low level of blood AA are important factors contributing to the development of chronic degenerative disorders.
As a further parallelism, the hypothesis proposed in the present paper also fits well with the Warburg theory postulating that cancer is originating from a metabolic dysfunction including insufficient mitochondrial oxidative phosphorylation and compensatorily enhanced glycolysis [93]. Cancer cells are highly dependent on glucose and are unable to use fat or ketones for energy [94], a metabolic failure ketogenic diets are aimed to exploit. We suggest that an animal based, low carbohydrate high fat diet (instead of the currently used ketogenic diets) may provide vitamin C not only in sufficient amounts but also in a bioavailable form, and thus may be more appropriate for the treatment of cancer as compared with the versions of the classical ketogenic diets that are currently used in clinical trials e.g. [95].
8. Conclusion
From the prospective cohort and correlational studies it is clear that higher blood level of AA is associated with lower mortality and morbidity in several chronic conditions. At the same time, high-grade evidence from clinical intervention studies indicate that vitamin C taken as a supplement provide little or no benefit in the prevention or treatment of chronic diseases. Indeed, apart from intervention studies of common cold where minor benefit was reported, no single RCT is available that found a clinically meaningful benefit in hard clinical endpoints of chronic diseases including cancer and cardiovascular diseases. We put forward that the discrepancy between correlational and interventional studies, as regards the role of vitamin C, is only apparent and may be resolved by introducing the GAA theory. As detailed above, metabolism of vitamin C, including absorption and its uptake by several cell types, is inhibited by increasing glucose concentration. Western type diets resulting in carbohydrate overconsumption and high blood level of glucose may inhibit utilization of vitamin C also when taken as a supplement. Current dietary guidelines, instead of animal sources, concentrate on plant sources of vitamin C, thereby also increasing carbohydrate load and intake of polyphenols which both inhibit vitamin C utilization. By contrast, studies of contemporary hunter-gatherer societies as well as documentations of the arctic people from the 18th and 19th century indicate no signs of scurvy despite subsisting on diets predominated by foods of animal origin and using no vitamin supplements. Our own clinical experience with the paleolithic ketogenic diet also shows improving health parameters and long-term sustainability of meat-fat based diet in the absence of vitamin C supplementation. It may be anticipated that supplementing vitamin C while on a Western type diet may not reproduce the biochemical and physiological complexity of the evolutionary adapted way to utilize vitamin C. Even though the amount of dietary vitamin C consumed on an animal meat-fat based diet may be lower as compared to dietary intake from some fruits and vegetables, the former may ensure a higher bioavailability of vitamin C. We believe that loss of vitamin C synthesis was not deleterious in the ancestral environment humans are evolutionary adapted to. Rather, a mismatch between our current diet and ancestral physiology may explain why deficient levels of vitamin C are associated with disease. Instead of supplementing vitamin C, changing our nutrition as a whole and adopting a meat-fat based diet, even if it may sound a radical solution, may be a better choice to support vitamin C homeostasis. Bearing in mind that ”nothing in biology makes sense except in the light of evolution” [96] this concept is in accordance with the major organizing principle of the living world: evolution.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
1. Foote, J.A., Murphy S.P., Wilkens L.R., Hankin J.H., Henderson B.E., Kolonel LN. Factors associated with dietary supplement use among healthy adults of five ethnicities: the Multiethnic Cohort Study. Am J Epidemiol 2003;157:888–97. http://aje.oxfordjournals.org/content/157/10/888.long
2. Willers J., Heinemann M., Bitterlich N. ,Hahn A. Vitamin Intake from Food Supplements in a German Cohort - Is there a Risk of Excessive Intake? Int J Vitam Nutr Res 2014;84:152–62. http://econtent.hogrefe.com/doi/pdf/10.1024/0300-9831/a000202
3. Hamel E.E., Santisteban G.A., Ely J.T. ,Read D.H. Hyperglycemia and reproductive defects in non-diabetic gravidas: a mouse model test of a new theory. Life Sci 1986;39:1425–8. http://www.ncbi.nlm.nih.gov/pubmed/3773637
4. Nobile S. ,Woodhill J.M. (1981) Vitamin C: the mysterious redox-system a trigger of life? MTP Press; Lancaster, Boston, 1981
5. Huang H.Y., Appel L.J., Croft K.D., Miller E.R. 3rd, Mori T.A. ,Puddey I.B. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: results of a randomized controlled trial. Am J Clin Nutr 2002;76:549–55. http://ajcn.nutrition.org/content/76/3/549.full
6. Yang J., Liu J., Parry J. Vitamin C: Daily Requirements, Dietary Sources and Adverse Effects. In: Handbook of Vitamin C Research. Eds: Kucharski H., Zajac J. Nova Science Publishers, Inc., 2009
7. Wilson J.X. The physiological role of dehydroascorbic acid. FEBS Lett.2002;527:5–9. http://onlinelibrary.wiley.com/doi/10.1016/S0014-5793(02)03167-8/epdf
8. Levine M., Conry-Cantilena C., Wang Y., Welch R.W., Washko P.W., Dhariwal K.R., Park J.B., Lazarev A., Graumlich J.F., King J, Cantilena L.R. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A 1996;93:3704–3709. http://www.pnas.org/content/93/8/3704.long
9. Malo C. ,Wilson J.X. Glucose modulates vitamin C transport in adult human small intestinal brush border membrane vesicles. J Nutr 2000;130:63–9. http://jn.nutrition.org/content/130/1/63.long
10. Wilson J.X. Regulation of vitamin C transport. Annu Rev Nutr 2005;25:105–25. http://www.annualreviews.org/doi/full/10.1146/annurev.nutr.25.050304.092647
11. Hediger M.A. New view at C. Nat Med 2002;8:445–6. http://www.nature.com/nm/journal/v8/n5/full/nm0502-445.html
12. Agus D.B., Gambhir S.S., Pardridge W.M., Spielholz C., Baselga J., Vera J.C, Golde D.W. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Inv 1997;100:2842–2848. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC508490/pdf/1002842.pdf
13. KC S., Cárcamo J.M., Golde D.W. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J 2005;19:1657–67. http://www.fasebj.org/content/19/12/1657.long
14. Olmedo J.M., Yiannias J.A., Windgassen E.B., Gornet M.K. Scurvy: a disease almost forgotten. Int J Dermatol 2006;45:909–13. http://onlinelibrary.wiley.com/doi/10.1111/j.1365-4632.2006.02844.x/full
15. Thomas L.D., Elinder C.G., Tiselius H.G., Wolk A., Akesson A. Ascorbic acid supplements and kidney stone incidence among men: a prospective study. JAMA Intern Med 2013;173:386–8. http://archinte.jamanetwork.com/article.aspx?articleid=1568519
16. Fritz H., Flower G., Weeks L., Cooley K., Callachan M., McGowan J., Skidmore B., Kirchner L., Seely D. Intravenous Vitamin C and Cancer: A Systematic Review. Integr Cancer Ther 2014;13:280–300. http://ict.sagepub.com/content/13/4/280.long
17. Kagawa Y., Higasa S., Tsujimura M., Komatsu F., Yanagisawa Y., Iwamoto S. Human Specific Vitamin C Metabolism and Xenobiotic Polymorphism: The Optimal Nutrition. In: Handbook of Vitamin C Research. Eds: Kucharski H, Zajac J. Nova Science Publishers, Inc., 2009
18. Ames B.N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 1981;78:6858–62. http://www.pnas.org/content/78/11/6858.full.pdf
19. Johnson, R. J., Andrews, P. Fructose, uricase, and the Back-to-Africa hypothesis. Evol Anthropol 2010;19:250–257. http://onlinelibrary.wiley.com/doi/10.1002/evan.20266/abstract?utm_sou
20. Schleicher R.L., Carroll M.D., Ford E.S., Lacher D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr 2009;90:1252–63. http://ajcn.nutrition.org/content/90/5/1252.long
21. Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington (DC): National Academies Press (US); 2000. Available from: http://www.ncbi.nlm.nih.gov/books/NBK225483
22. Fruit and vegetables for health. Report of a Joint FAO/WHO Workshop, 1–3 September 2004, Kobe, Japan
23. Fediuk K. Vitamin C in the Inuit diet: past and present. MA Thesis, School of Dietetics and Human Nutrition, McGill University, 2000
24. Fediuk K., Hidiroglou N., Madère R., Kuhnlein H.V. Vitamin C in Inuit Traditional Food and Women's Diets. J Food Comp Anal 2002;15:221–235. http://www.sciencedirect.com/science/article/pii/S0889157502910537
25. Voegtlin V.L. The Stone Age Diet. Vantage Press, New York, 1975
26. Cordain L., Miller J.B., Eaton S.B., Mann N., Holt S.H. and Speth J.D. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am J Clin Nutr 2000;71:682–92. http://ajcn.nutrition.org/content/71/3/682.full.pdf+html
27. Cordain L., Eaton S.B., Miller J.B., Mann N. and Hill K. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr 2002;56,Suppl 1:S42–52. http://www.nature.com/ejcn/journal/v56/n1s/abs/1601353a.html
28. O'Dea K. Traditional diet and food preferences of Australian aboriginal hunter-gatherers. Philos Trans R Soc Lond B Biol Sci 1991;334,233–40. http://www.jstor.org/stable/55460?seq=1#page_scan_tab_contents
29. Geraci J.R., Smith T.G. Vitamin C in the diet of inuit hunters from Holman northwest territories. Arctic 1979;32:135–139. http://pubs.aina.ucalgary.ca/arctic/Arctic32-2-135.pdf
30. McClellan W.S., Du Bois E.F. Clinical calorimetry. XLV. Prolonged meat diets with a study on kidney function and ketosis. 1930;87:651–668. http://www.jbc.org/content/87/3/651.full.pdf
31. Lindeberg S, Eliasson M, Lindahl B, Ahrén B. Low serum insulin in traditional Pacific Islanders--the Kitava Study. Metabolism. 1999;48:1216–9. http://www.metabolismjournal.com/article/S0026-0495(99)90258-5/abstract
32. Clemens Z., Kelemen A., Fogarasi A., Tóth C. Childhood absence epilepsy successfully treated with the paleolithic ketogenic diet. Neurol Ther 2013;2:71–6. http://link.springer.com/article/10.1007%2Fs40120-013-0013-2#/page-1
33. Tóth C., Clemens Z. Type 1 diabetes mellitus successfully managed with the paleolithic ketogenic diet. Int J Case Rep Images 2014;5:699–703. http://www.ijcasereportsandimages.com/archive/2014/010-2014-ijcri/CR-10435-10-2014-clemens/index.php
34. Tóth C., Clemens Z. Successful treatment of a patient with obesity, type 2 diabetes and hypertension with the paleolithic ketogenic diet. Int J Case Rep Images 2015;6:161–167. http://www.ijcasereportsandimages.com/archive/2015/003-2015-ijcri/CR-10491-03-2015-toth/ijcri-1049103201591-toth.pdf
35. Tóth C., Clemens Z. Gilbert’s syndrome successfully treated with the paleolithic ketogenic diet. Am J Med Case Rep 2015;3:117–120. http://pubs.sciepub.com/ajmcr/3/4/9/
36. Clemens, Z., Kelemen, A., Tóth, C. NREM-sleep Associated Epileptiform Discharges Disappeared Following a Shift toward the Paleolithic Ketogenic Diet in a Child with Extensive Cortical Malformation. Am J Med Case Rep 2015;3:212–215. http://pubs.sciepub.com/ajmcr/3/7/8/
37. Willmott N.S., Bryan R.A. Case report: scurvy in an epileptic child on a ketogenic diet with oral complications. Eur Arch Paediatr Dent 2008;9:148–52. http://link.springer.com/article/10.1007/BF03262627#/page-1
38. Dehghan M., Akhtar-Danesh N., McMillan C.R., Thabane L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutrition Journal 2007;6:41. http://link.springer.com/article/10.1186%2F1475-2891-6-41
39. Khaw K.T., Bingham S., Welch A., Luben R., Wareham N., Oakes S., Day N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European prospective investigation into cancer and nutrition. Lancet 2001;3:57, :657–63. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(00)04128-3/abstract
40. Loria C.M., Klag M.J., Caulfield L.E. and Whelton P.K. Vitamin C status and mortality in US adults. Am J Clin Nutr 2000;72:139–45. http://ajcn.nutrition.org/content/72/1/139.long
41. Fletcher AE, Breeze E, Shetty PS. Antioxidant vitamins and mortality in older persons: findings from the nutrition add-on study to the Medical Research Council Trial of Assessment and Management of Older People in the Community. Am J Clin Nutr 2003;78:999–1010. http://ajcn.nutrition.org/content/78/5/999.long
42. Gale C.R., Martyn C.N., Winter P.D., Cooper C. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. BMJ 1995;310:1563–6. http://www.bmj.com/content/310/6994/1563
43. Gey K.F., Stähelin H.B., Eichholzer M. Poor plasma status of carotene and vitamin C is associated with higher mortality from ischemic heart disease and stroke: Basel Prospective Study. Clin. Investig. 1993;71:3–6. http://link.springer.com/article/10.1007/BF00210955
44. Deicher R., Ziai F., Bieglmayer C., Schillinger M., Hörl W.H. Low total vitamin C plasma level is a risk factor for cardiovascular morbidity and mortality in hemodialysis patients. J Am Soc Nephrol 2005;16:1811–8. http://jasn.asnjournals.org/content/16/6/1811.full.pdf
45. Simon J.A., Hudes E.S., Tice J.A. Relation of serum ascorbic acid to mortality among US adults. J Am Coll Nutr 2001;20:255–63. http://www.tandfonline.com/doi/abs/10.1080/07315724.2001.10719040?journalCode=uacn20
46. Jenab M., Riboli E., Ferrari P., Sabate J., Slimani N., Norat T., Friesen M., Tjønneland A., Olsen A., Overvad K., Boutron-Ruault M.C., Clavel-Chapelon F., Touvier M., Boeing H., Schulz M., Linseisen J., Nagel G., Trichopoulou A., Naska A., Oikonomou E., Krogh V., Panico S., Masala G., Sacerdote C., Tumino R., Peeters P.H., Numans M.E., Bueno-de-Mesquita H.B., Büchner F.L., Lund E., Pera G., Sanchez C.N., Sánchez M.J., Arriola L., Barricarte A., Quirós J.R., Hallmans G., Stenling R., Berglund G., Bingham S., Khaw K.T., Key T., Allen N., Carneiro F., Mahlke U., Del Giudice G., Palli D., Kaaks R.and Gonzalez C.A. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 2006;27:2250–7. http://carcin.oxfordjournals.org/content/27/11/2250.long
47. Mayland C.R. Bennett M.I., Allan K. Vitamin C deficiency in cancer patients. Palliat Med 2005;19:17–20. http://pmj.sagepub.com/content/19/1/17.abstract
48. Simon J.A., Hudes E.S., Browner W.S. Serum ascorbic acid and cardiovascular disease prevalence in U.S. adults. Epidemiology 1998;9:316–21. http://www.jstor.org/stable/3703063?seq=1#page_scan_tab_contents
49. Myint P.K., Luben R.N., Welch A.A., Bingham S.A., Wareham N.J., Khaw K.T. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Am J Clin Nutr 2008;87:64–9. http://ajcn.nutrition.org/content/87/1/64.long
50. Ness A.R., Khaw K.T., Bingham S., Day N.E. Vitamin C status and blood pressure. J Hypertens 1996;14:503–8. http://www.ncbi.nlm.nih.gov/pubmed/8761901
51. Block G., Jensen C.D., Norkus E.P., Hudes M. and Crawford P.B. Vitamin C in plasma is inversely related to blood pressure and change in blood pressure during the previous year in young black and white women. Nutr J 2008;7:35. https://nutritionj.biomedcentral.com/articles/10.1186/1475-2891-7-35
52. Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 2012;3:CD007176. http://dx.doi.org/10.1002/14651858.CD007176.pub2
53. Hemilä H., Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD000980.pub4/full
54. Cook N.R., Albert C.M., Gaziano J.M., Zaharris E., MacFadyen J., Danielson E., Buring J.E., Manson J.E. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch. Intern. Med. 2007;167:1610–8. http://archinte.jamanetwork.com/article.aspx?articleid=769855
55. Sesso H.D., Buring J.E., Christen W.G., Kurth T., Belanger C., MacFadyen J., Bubes V., Manson J.E., Glynn R.J., Gaziano J.M. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–33. http://jama.jamanetwork.com/article.aspx?articleid=1028653
56. Juraschek S.P., Guallar E., Appel L.J. and Miller ER 3rd. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012;95:1079–88. http://ajcn.nutrition.org/content/early/2012/04/03/ajcn.111.027995.full.pdf+html
57. Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 1976;73:3685–9. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC431183
58. Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A 1978;75:4538–4542. http://www.pnas.org/content/75/9/4538.full.pdf
59. Cameron E, Campbell A. Innovation vs. quality control: an 'unpublishable' clinical trial of supplemental ascorbate in incurable cancer. Med Hypotheses 1991;36:185–9. http://www.medical-hypotheses.com/article/0306-9877(91)90127-K/abstract
60. Creagan E.T., Moertel C.G., O'Fallon J.R., Schutt A.J., O'Connell M.J., Rubin J., Frytak S. Failure of high-dose vitamin C (ascorbic acid): therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 1979;301,687–90. http://www.nejm.org/doi/full/10.1056/NEJM197909273011303
61. Moertel C.G., Fleming T.R., Creagan E.T., Rubin J, O'Connell M.J., Ames M.M. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med 1985;312:137–41. http://www.nejm.org/doi/full/10.1056/NEJM198501173120301
62. Wang L., Sesso H.D., Glynn R.J., Christen W.G., Bubes V., Manson J.E., Buring J.E., Gaziano J.M. Vitamin E and C supplementation and risk of cancer in men: posttrial follow-up in the Physicians' Health Study II randomized trial. Am J Clin Nutr 2014;100:915–23. http://ajcn.nutrition.org/content/early/2014/07/09/ajcn.114.085480.abstract
63. Lin J., Cook N.R., Albert C., Zaharris E., Gaziano J.M., Van Denburgh M., Buring J.E. and Manson J.E. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst 2009;101:14–23. http://jnci.oxfordjournals.org/content/101/1/14.long
64. Coulter I.D., Hardy M.L., Morton S.C., Hilton L.G., Tu W., Valentine D., Shekelle P.G. Antioxidants vitamin C and vitamin e for the prevention and treatment of cancer. J Gen Intern Med 2006;21:735–44. http://link.springer.com/article/10.1111%2Fj.1525-1497.2006.00483.x
65. Leung P.Y., Miyashita K., Young M., Tsao C.S. Cytotoxic effect of ascorbate and its derivatives on cultured malignant and nonmalignant cell lines. Anticancer Res. 1993;13:475–80. http://www.ncbi.nlm.nih.gov/pubmed/8517665
66. Padayatty S.J., Sun H., Wang Y., Riordan H.D., Hewitt S.M., Katz A., Wesley R.A., Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 2004;140:533–7. http://annals.org/article.aspx?articleid=717329
67. Chen Q., Espey M.G., Krishna M.C., Mitchell J.B., Corpe C.P., Buettner G.R., Shacter E., Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. U S A. 2005;102:13604–9. http://www.pnas.org/content/102/38/13604.long
68. Mojić M., Pristov J.B., Maksimović-Ivanić D., Jones D.R., Stanić M., Mijatović S., Spasojević I. Extracellular iron diminishes anticancer effects of vitamin C: An in vitro study. Scientific Reports. 2014;4:5955. http://www.nature.com/articles/srep05955
69. Ma Y., Chapman J., Levine M., Polireddy K., Drisko J., Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med 2014;6:222ra18. http://stm.sciencemag.org/content/6/222/222ra18.long
70. Monti D.A., Mitchell E., Bazzan A.J., Littman S., Zabrecky G., Yeo C.J., Pillai M.V., Newberg A.B., Deshmukh S. and Levine M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One 2012;7:e29794. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0029794
71. Stephenson C.M., Levin R.D., Spector T., Lis C.G. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol 2013;72:139–46. http://link.springer.com/article/10.1007%2Fs00280-013-2179-9
72. Mikirova N., Casciari J., Rogers A., Taylor P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med 2012;10:189. http://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-10-189
73. Welsh J.L., Wagner B.A., van't Erve T.J., Zehr P.S., Berg D.J., Halfdanarson T.R., Yee N.S., Bodeker K.L., Du J., Roberts L.J. Drisko J., Levine M., Buettner G.R., Cullen J.J. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol 2013;71:765–75. http://link.springer.com/article/10.1007%2Fs00280-013-2070-8
74. Hoffer L.J., Levine M., Assouline S., Melnychuk D., Padayatty S.J., Rosadiuk K., Rousseau C., Robitaille L., Miller W.H. Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol 2008;19:1969–74. http://annonc.oxfordjournals.org/content/early/2008/07/25/annonc.mdn377
75. Riordan H.D., Casciari J.J., González M.J., Riordan N.H., Miranda-Massari J.R., Taylor P., Jackson J.A. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J 2005;24: 269–76. http://www.ncbi.nlm.nih.gov/pubmed/16570523
76. Yeom C.H., Jung G.C. and Song K.J. Changes of terminal cancer patients' health-related quality of life after high dose vitamin C administration. J Korean Med Sci 2007;22:7–11. http://jkms.org/DOIx.php?id=10.3346/jkms.2007.22.1.7
77. Hoffer LJ, Robitaille L, Zakarian R, Melnychuk D, Kavan P, Agulnik J, Cohen V, Small D, Miller WH Jr. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: a phase I-II clinical trial. PLoS One 2015;10:e0120228. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0120228
78. Riordan H.D., Riordan N.H., Jackson J.A., Casciari J.J., Hunninghake R., González M.J., Mora E.M., Miranda-Massari J.R., Rosario N., Rivera A. Intravenous vitamin C as a chemotherapy agent: a report on clinical cases. P R Health Sci J 2004;23:115–8. http://www.ncbi.nlm.nih.gov/pubmed/15377059
79. Cunningham J.J., Ellis S.L., McVeigh K.L., Levine R.E., Calles-Escandon J. Reduced mononuclear leukocyte ascorbic acid content in adults with insulin-dependent diabetes mellitus consuming adequate dietary vitamin C. Metabolism 1991;40:146–9. http://www.ncbi.nlm.nih.gov/pubmed/1988772
80. Fadupin G.T., Akpoghor A.U., Okunade K.A. A comparative study of serum ascorbic acid level in people with and without type 2 diabetes in Ibadan, Nigeria. Afr J Med Med Sci 2007;36:335–9. http://www.ncbi.nlm.nih.gov/pubmed/18564649
81. Varma V., Varma M., Sarkar P.D., Varma A., Vyas S. and Kulkarni R. Correlation of vitamin C with HbA1c and oxidatice stress in diabetes mellitus with or without nephropathy. Natl J Med Res 2014;4:151–155. http://www.scopemed.org/?mno=162318
82. Seghieri G., Martinoli L., Miceli M., Ciuti M., D'Alessandri G., Gironi A., Palmieri L., Anichini R. Bartolomei G., Franconi F. Renal excretion of ascorbic acid in insulin dependent diabetes mellitus. Int J Vitam Nutr Res 1994;64:119–24. http://www.ncbi.nlm.nih.gov/pubmed/7960490
83. Rivas C.I., Zúñiga F.A., Salas-Burgos A., Mardones L., Ormazabal V., Vera J.C. Vitamin C transporters. J Physiol Biochem 2008;64:357–75. http://link.springer.com/article/10.1007/BF03174092
84. Ng L.L., Ngkeekwong F.C., Quinn P.A., Davies JE. Uptake mechanisms for ascorbate and dehydroascorbate in lymphoblasts from diabetic nephropathy and hypertensive patients. Diabetologia 1998;41:435–42. http://link.springer.com/article/10.1007%2Fs001250050927
85. Chen M.S., Hutchinson M.L., Pecoraro R.E., Lee W.Y., Labbé R.F. Hyperglycemia-induced intracellular depletion of ascorbic acid in human mononuclear leukocytes. Diabetes 1983;32:1078–81. http://diabetes.diabetesjournals.org/content/32/11/1078.long
86. Choi M.K., Song H.J., Paek Y.J., Lee H.J. Gender differences in the relationship between vitamin C and abdominal obesity. Int J Vitam Nutr Res 2013;83:377–84. http://econtent.hogrefe.com/doi/pdf/10.1024/0300-9831/a000179
87. Krone C.A., Ely J.T. Controlling hyperglycemia as an adjunct to cancer therapy. Integr Cancer Ther 2005;4:25–31. http://ict.sagepub.com/content/4/1/25.long
88. Johnstone A.M., Lobley G.E., Horgan G.W., Bremner D.M., Fyfe C.L., Morrice P.C., Duthie G.G. Effects of a high-protein, low-carbohydrate v. high-protein, moderate-carbohydrate weight-loss diet on antioxidant status, endothelial markers and plasma indices of the cardiometabolic profile. Br J Nutr 2011;106:282–91. http://www.ncbi.nlm.nih.gov/pubmed/21521539
89. Mobbs C.V., Mastaitis J.W., Zhang M., Isoda F., Cheng H., Yen K. Secrets of the lac operon. Glucose hysteresis as a mechanism in dietary restriction, aging and disease. Interdiscip. Top Gerontol 2007;35:39–68. http://www.karger.com/Article/Abstract/96555
90. Song J., Kwon O., Chen S., Daruwala R., Eck P., Park J.B., Levine M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and Glucose. J Biol Chem 2002;277:15252–60. http://www.jbc.org/content/277/18/15252.long
91. Westman E.
 Rehabilitáció csak online elérhető
Rehabilitáció csak online elérhető
 E-mail: paleomedicina@gmail.com
E-mail: paleomedicina@gmail.com


